This is a Food Safety Management System Standard
Hazard Analysis Critical Control Points (HACCP) is an internationally recognized standard and method of identifying and managing food safety related risk, as an assurance that the Organization can provide to customers, the public, and regulatory agencies assurance that a food safety program / management system has been established and is being well managed.
HACCP is a management system standard / set of Principles / set of Guidelines in which food safety is addressed through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, storage, to manufacturing, distribution and including the consumption of the finished product.

Why is HACCP Important?
(including the standard developed by Euro Veritas (accreditated from BAR-UK)- EVL/HACCP/C-A/3788/C-6 )
Systematic documentation and implementation of a HACCP program helps reduce and control the likelihood of customer complaints or a recall by identifying and controlling potential hazards which may come from raw materials (including at the suppliers’ place), facility processes, and human error. The greater employee awareness that results from a HACCP program helps to drive continual improvement of a company’s products and processes.
Additionally, it should be noted that the HACCP principles are in alignment with the requirements of the FDA’s Food Safety Modernization Act (FSMA) rule for food processors — Hazard Analysis and Risk-based Preventive Controls (“HARPC” or “Preventive Controls”). Although a HACCP plan does not meet all of the requirements, it meets the majority of the requirements and is the best platform from which to build a FSMA-compliant management system.
Euro Veritas, UK (www.euroveritas.com) accreditated from BAR-UK has developed a Procedure of their own- EVL/HACCP/C-A/3788/C-6 against which it audits and certified any organization against HACCP.
Is HACCP right for my organization?
(including the standard developed by Euro Veritas (accreditated from BAR-UK)- EVL/HACCP/C-A/3788/C-6 )
Hazard Analysis and Critical Control Points (HACCP) certification instantly demonstrates to customers your commitment to producing or trading in safe food. This evidence-based approach can be particularly beneficial when you are subjected to inspection by regulatory authorities or stakeholders.
An [Euro Veritas, UK (www.euroveritas.com) accreditated from BAR-UK] HACCP certificate will give your business improved credibility and recognition and assurance due to the brand image and market reputation of Euro Veritas, UK (www.euroveritas.com) accreditated from
The Certificate from Euro Veritas, UK (www.euroveritas.com) accreditated from BAR-UK can be used by any organization directly or indirectly involved in the food chain including food service providers (canteens, restaurants, fast food chains, caterers, hospitals, hotels, etc.), farms, fisheries, dairies, processors of food for both human and livestock consumption and supporting services such as transporters and distributors.
Organizations that implement and get certified to HACCP Standard must comply with GMP, Good Manufacturing Practices, as they are a mandatory requirement included within the HACCP standard. However, it is possible to certify each individually.
HACCP PRINCIPLES
HACCP (including the standard developed by Euro Veritas (accreditated from BAR-UK)- EVL/HACCP/C-A/3788/C-6 ) is a systematic approach to the identification, evaluation, and control of food safety hazards based on the following seven principles:
• Principle 1: Conduct a hazard analysis.
• Principle 2: Determine the critical control points (CCPs).
• Principle 3: Establish critical limits.
• Principle 4: Establish monitoring procedures.
• Principle 5: Establish corrective actions.
• Principle 6: Establish verification procedures.
• Principle 7: Establish record-keeping and documentation procedures.
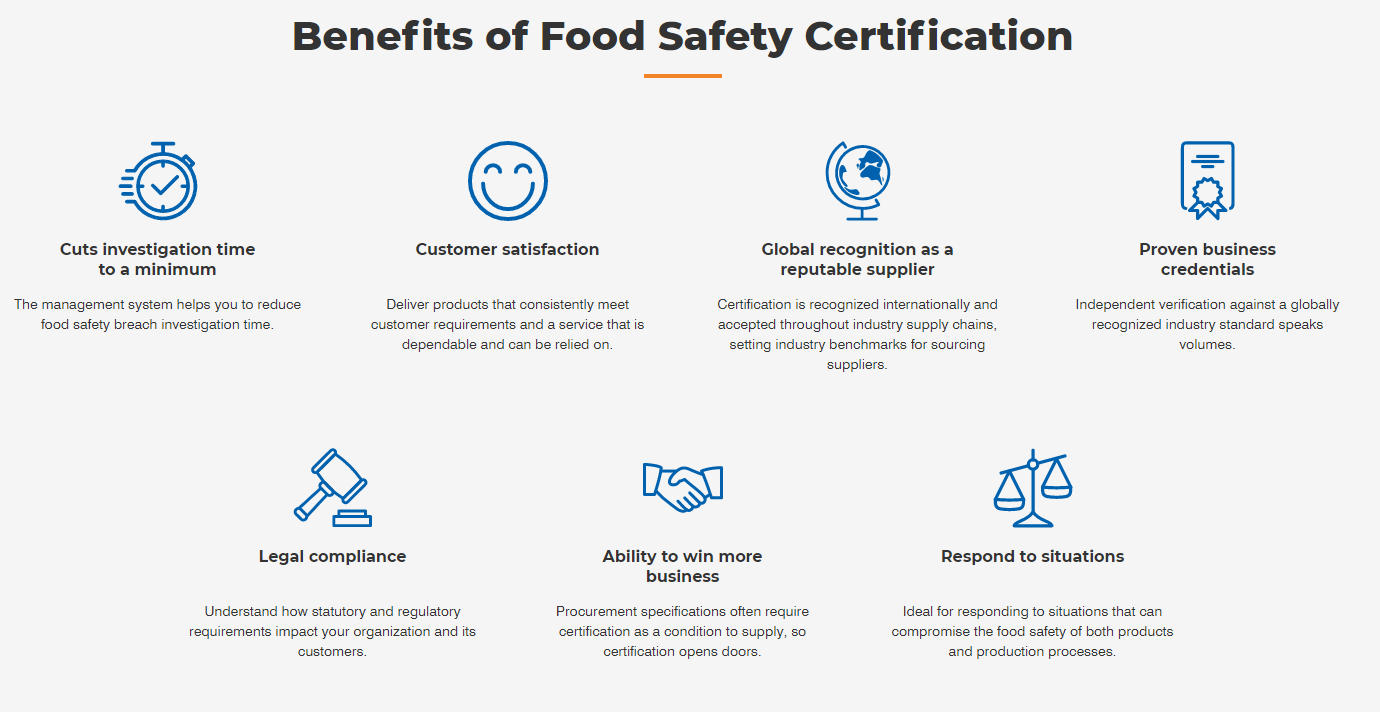
Benefits of HACCP certification
(including the standard developed by Euro Veritas (accreditated from BAR-UK)- EVL/HACCP/C-A/3788/C-6 )
HACCP based procedures- EVL/HACCP/C-A/3788/C-6 provide businesses with a cost effective system for control of food safety, from ingredients right through to production, storage and distribution to sale and service of the final consumer. The preventive approach of HACCP based procedures not only improves food safety management but also complements other quality management systems. The main benefits of HACCP based procedures are:
• Saves your business money in the long run
• Avoids you contaminating your customers
• Food safety standards improvement
• Ensures you are compliant with the law
• Food quality standards increase
• Organizes your process to produce safe food
• Organizes your staff promoting teamwork and efficiency
• Due diligence defense in court.
The primary purpose of a HACCP system is to protect people from food borne illness, but the benefits of the system also extend to the company.
• Increased confidence in your products
• Ability to reach markets and customers that require a HACCP based system
• Reduced Liability
• Effective process management
• Improved quality and consistency
• Encourages a more effective use of your resources
• Reduces costs by avoiding unsafe productions
• Enables your company to act quickly and effectively in the event of a food safety emergency
• Builds customer and health authorities trust
• Reduces barriers to international trade
• Helps your food company competes more effectively in the world market
• Work with us to achieve HACCP compliance and meet the expectations of a changing world.
GUIDELINES FOR APPLICATION OF HACCP PRINCIPLES
• Develop Prerequisite Programs
• Education and Training to Employees and Personnel
• Developing a HACCP Plan
• Assemble the HACCP team
• Describe the food and its distribution
• Describe the intended use and consumers of the food
• Develop a flow diagram which describes the process
• Verify the flow diagram
• Conduct a hazard analysis (Principle 1)
• Determine critical control points (CCPs) (Principle 2)
• Establish critical limits (Principle 3)
• Establish monitoring procedures (Principle 4)
• Establish corrective actions (Principle 5)
• Establish verification procedures (Principle 6)
• Establish record-keeping and documentation procedures (Principle 7)
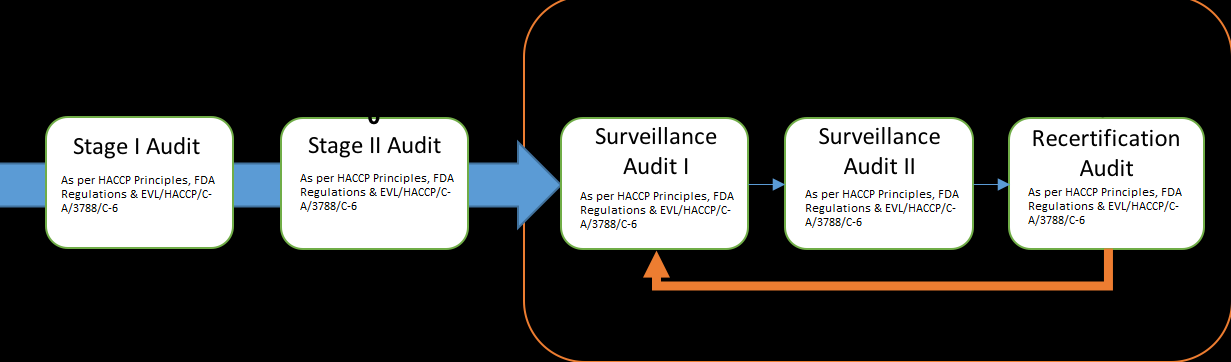
Certification to HACCP- this is as per the requirements of EVL/HACCP/C-A/3788/C-6
All elements of HACCP EVL/HACCP/C-A/3788/C-6 should be set in consecutive flow so that when consulted or reviewed, everything is aligned to the methodology.
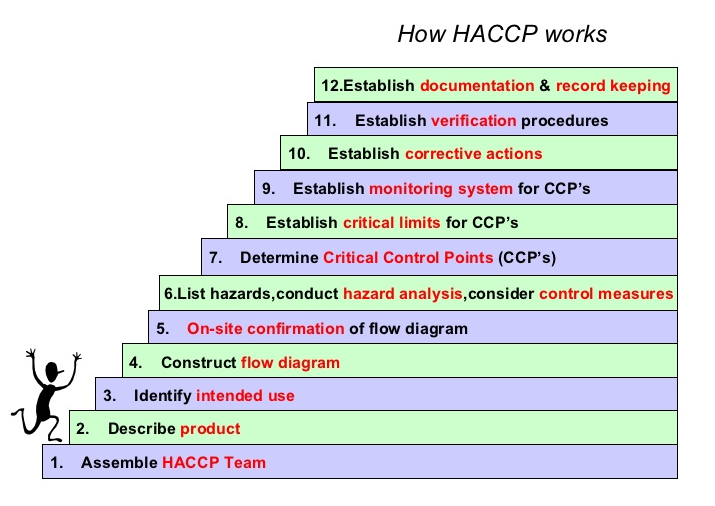
The first element in a HACCP plan is contains all the names of the HACCP Team members, and the team leader is clearly appointed. A brief description of each member’s current position, expertise and experience should be included. Responsibilities, Authorities, Accountabilities of all personnel to be defined here. HACCP formal training certificate (Type 1, Type 2, Type 3 and Type 4) of at least one team member should be included. The more knowledgeable the team, the better plan will result.
The second item in the plan contains that a manual is made and that there should be a full description of the product, products or family of products within the scope of the plan. The description should include the recipe or formulation, when applicable. The packing materials should also be described as well as the conditions in which the product is to be stored (e.g. temperature, light, humidity) and the length of shelf life. In the description, methods of distribution should be addressed, too; taking into consideration the potential abuse in the distribution chain as well as on behalf of the consumers. The more you know your product, the better you can protect it.
The third component in the plan is the identification of the intended use and consumers. Identification of the intended use must be based on the “usual” consumption of the commodity by the final consumer or user. The identification of consumers shall be based on the targeted consumer. It could be just a segment of the population, such as diabetic individuals, the elderly, infants, individuals allergic or intolerant to a certain food, immunologically compromised people, etc. The more you know your consumers, the better care you can take of them.
The forth constituent in the plan is the flow diagram. The diagram has to be clear and in enough detail to provide a simple description of the process steps. This diagram is very helpful to the team for future tasks and to others (e.g. customers, auditors, consultants, regulatory officials). Adding some process parameters (e.g. temperature, time, etc.) for each step could be useful, too. The flow diagram must comprehend every process step which is in direct control of the facility manufacturing the food, from the very beginning (e.g. receiving and preparing ingredients, storing packing materials, etc.) to the very end (shipping, storing, etc.) Using engineering drawing is not recommended. The friendlier the diagram is to the viewer, the easier to understand the process.
The fifth element in the plan should be proof that the HACCP Team has verified the flow diagram. This could be the first version of the diagram where notes have been taken and changes made, initialized and dated by the participants. Proof of the verification could also be minutes of the verification process and changes made to the original. The new verified diagram must be available. When no inaccuracies were detected in the original diagram by the verification process, a signed and dated note stating that fact shall be available. The more accurate the diagram, the more useful it’ll be.
The sixth part of the plan must be the documented hazard analysis. This document must make evident the hazard significance assessment based on likelihood vs. severity. The consideration of a hazard being significant or not shall be supported by a justification. Non-significant hazards are not expected to be further considered. However, each hazard (physical, chemical or biological) found to be significant must have a linked preventive measure (e.g. GMPs, a prerequisite program) specific for its control. In the hazard analysis, it is expected to state the specific and actual hazard; and not the cause or the source of it (e.g. Salmonella, glass, sodium benzoate, etc.) The sequence of the analyzed steps has to be the same as that of the verified flow diagram. The more preventive measures, the fewer CCP may be determined.
The seventh portion of the plan should contain documents evidencing the CCP determination process. In this document, all hazards found to be significant have to be addressed. The method used for CCPs determinations shall be available, too (e.g. decision tree, questioner, etc.) CCPs found have to be documented.
The eighth section of the plan should contain the critical limits that were determined for each particular CCP. All the variables, values and units have to be clearly defined; for both lower and upper limits, when applicable. Documents related to the process and relevant sources used to establish the critical limits shall be available to support the limits. These documents could be regulatory standards, guidelines, internal or third party validation, experimental results, literature surveys or experts. The stricter the validated limits, the grater efficacy can be achieved.
The ninth segment of the plan should comprise of documents regarding the monitoring method for each critical limit. The monitoring procedure should contain the following: what is to be monitored? How often it shall be monitored? Who is responsible for performing the task? What instruments are to be used? How is to be monitored? The clearer the instructions are the fewer chances of failure.
The tenth element in the plan should be the corrective actions strategy. Each CCP should have predetermined and documented corrective actions for deviations that may occur. The corrective actions plan should comprise at least the following elements: the responsibility for each action, disposition of the non-complying product, the correction of the cause of failure and recording the event. Records of events should be kept readily available. The more preparation, the less improvisation.
The eleventh component of the plan should be the HACCP Plan verification procedures. These procedures should be content activities designed to confirm that the plan is effective and properly maintained. Responsibility and frequency shall be defined. Some of the following elements should be part of the verification process: description of auditing methods, product sampling and testing, trending analysis, reviewing records and reviewing the whole plan. The more exhaustive the verification is the grater the confidence.
The twelfth module of the plan should comprehend the record keeping and documentation system. In this section it should respond to the following questions? How the system is to be documented? What is to be included? Who is responsible for doing it? For how long are records to be kept? Where are they going to be kept? Who is to have access to what documents and how are those to be controlled?
A more documented plan helps with better execution and during auditing and certification.
HACCP is an internationally accepted method of preventing food contamination from chemical, microbiological and physical hazards.
Hazard Analysis Critical Control Point (HACCP), is the internationally recognized food safety system that focuses on the systems needed to identify and manage potential risks of foodborne safety hazards from reaching consumers. It pertains to any organization involved directly or indirectly in the food chain such as farms, fisheries, dairies, meat processors, and food service providers such as restaurants, hospitals, and catering services.
HACCP is a risk management system designed to identify, assess, evaluate and control hazards in the entire food production process. Audits focus on the risks and potential causes of food safety hazards and utilizes preventative controls at critical points. Critical control points may include the supply chain, employee training and supervision, storage, preparation, handling, cooking to serving. It specifies how each should be carried out and meticulously monitored.
The HACCP Certification against EVL/HACCP/C-A/3788/C-6 has international recognition as one of the most cost-effective means of controlling food borne disease and is endorsed as such by the Joint FAO/WHO Codex Alimentarius Commission.
A strategic and properly implemented HACCP program can reduce the likelihood of customer complaints or costly recalls and provide your customers, the public, and regulatory agencies assurance that a food safety program is well-managed.
Euro Veritas, UK (www.euroveritas.com) accreditated from BAR-UK has developed a Standard- Procedure of their own- EVL/HACCP/C-A/3788/C-6 against which it audits and certified any organization against HACCP.
In the entire section above, and the certification issuance from Euro Veritas (accreditated from BAR-UK), the term- HACCP automatically means the standard developed by Euro Veritas (accreditated from BAR-UK)- EVL/HACCP/C-A/3788/C-6.


